UHC Laboratory Test Code Registry – Delayed
UPDATE (7/1/2021) – UnitedHealthcare is delaying the implementation of the clinical and pathology Laboratory Test Registry Protocol until further notice. This includes registration of non-genetic tests and placement of test codes on claims for non-genetic tests. There is no need to register non-genetic tests at this time.
Laboratory Test Registry Protocol
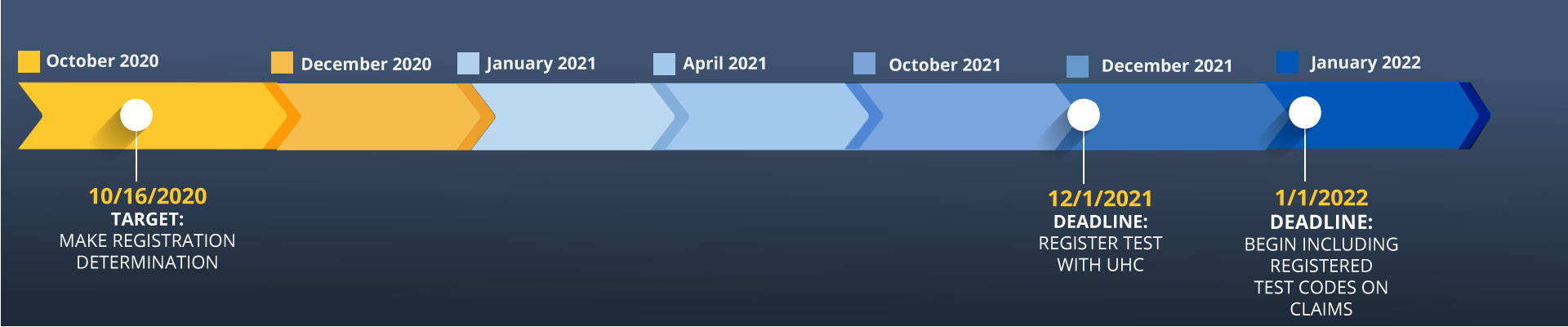
Effective January 1, 2022, UnitedHealthcare (UHC) will require in-network, freestanding, and outpatient hospital laboratories to include the laboratory’s unique test code on claims for most laboratory test services. Each test code submitted on a claim must match a corresponding laboratory test registration provided in advance to UHC, or the claim will be denied.
Meet Compliance Requirements with Quadax
Registration Determination
To ensure compliance, laboratory providers subject to these requirements must self-register their tests prior to December 1, 2021. These requirements apply to most UHC Commercial, Medicare Advantage and UHC Community Plan networks. As defined by UHC, a laboratory test code is the lab’s unique identifier used by a physician to order a test. UHC is not specifying which values or coding method to use to uniquely identify tests. Labs are free to use their own test codes as long as the codes are in the UHC registry.
We have outlined the UHC strategies for test registration in the white paper Quadax UHC Laboratory Test Registration: How to Prepare and Comply, which can assist clients with each step of the registration determination process, including sending test codes to Quadax and lab code claim criteria details.